Sialic acid
 Sialic acid |
Sialic acid is a generic term for the N-or O-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone. It is also the name for the most common member of this group, N-acetylneuraminic acid (Neu5Ac or NANA). Sialic acids are found widely distributed in animal tissues and to a lesser extent in other species ranging from plants and fungi to yeasts and bacteria, mostly in glycoproteins and gangliosides. The amino group generally bears either an acetyl or glycolyl group but other modifications have been described. The hydroxyl substituents may vary considerably: acetyl, lactyl, methyl, sulfate, and phosphate groups have been found. The term "sialic acid" (from the Greek for saliva, σ?αλον/sialon) was first introduced by Swedish biochemist Gunnar Blix in 1952. |
| Contents |
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
Product: N-Acetylneuraminic acid |
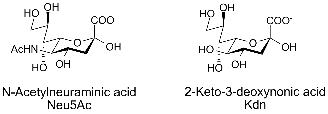 The numbering of the sialic acid structure begins at the carboxylate carbon and continues around the chain. The configuration which places the carboxylate in the axial position is the alpha-anomer. |
|
|
||||
|
Form |
crystalline |
|
Hazard Code |
Xi |
|
mp |
184-186 °C (dec.)(lit.) |
|
Risk Phrases |
36/37/38 |
|
Specific Rotation |
-32 o (C=2,water) |
|
Safety Phrases |
22-24/25-36-26 |
|
Index of Refraction |
-32 ° (C=1, H2O) |
|
WGK Germany |
3 |
|
Solubility |
50 g/L H2O (20°C) |
|
F |
3-10-23 |
|
Sensitivity |
Air Sensitive |
|
Hazard Note |
Irritant |
|
Storage |
?20°C |
|
HS Code |
29329970 |
|
|
|
|
|
|
|
It plays a very important role in the production and development of brain and nervous system. Researches show that decreasing ganglioside has something to do with early malnutrition and learning disability. A proper complement of sialic acid is good either to behavior learning improvement or early brain development. |
Seebio Biotech, Inc. provides N-Acetylneuraminic acid as follows.
| Code | Product | CASN | Grade |
| DLK0011A | N-Acetylneuraminic acid (Sialic acid) | 131-48-6 | ≥99.0% |
11-502. Lane 299, Bisheng Rd.
Zhangjiang High Tech Park
Shanghai 201204, P.R.China
Tel: 86-21-50272975, 76, 77, 78, 79, 81; Ext:6513, 6515, 6516, 6520, 6521, 6522
For customers' complaint or suggestion: market5@seebio.cn
Web: www.seebio.cn
Email: fyland@seebio.cn; service@seebio.cn
MSN:seebio8@hotmail.com
Skype:seebiocn
 |
 |
 |
| 官網:www.baichuan365.com | 微信服務號:iseebio | 微博:seebiobiotech |
 |
 |
 |
| 商城:mall.seebio.cn | 微信訂閱號:seebiotech | 泉養堂:www.canmedo.com |
相關資訊
- Sigma Supelco 離子液體氣相毛細管色譜柱
- 如何使用抗-PEG瓊脂糖親和純化mPEG-BSA
- 科學家有望利用鼻細胞成功治療人類脊髓損傷
- α-酮戊二酸|α-Ketoglutaric acid|328-50-7|西寶生物
- Cancer Discovery:KRAS誘導線粒體自噬來促進胰腺癌發展
- 藥用輔料 - 卡波姆(carpol)
- 美國多家診斷公司獲允提供院外診斷服務
- Miscellaneous 其它低聚糖 - Elicityl細菌發酵生產低聚葡聚糖產品 (9)
- 納斯達克生物科技股的泡沫來了嗎?
- Cell:重磅!揭示免疫檢查點抑制劑攻擊癌癥機制
新進產品
同類文章排行
- 膽堿酯酶檢測原料一覽
- 猴痘病毒檢測原料(涵蓋診斷酶、抗原、抗體),西寶生物助力猴痘檢測
- 西寶生物CHO細胞、HEK293細胞、 Vero細胞培養基產品
- 人和動物免疫球蛋白IgG全新上市
- 如何選擇合適的表面活性劑?
- 尿液分析試紙檢測原理及產品推薦
- 總膽紅素/直接膽紅素檢測一站式解決方案
- 分子診斷用酶原料一站式服務盡在西寶生物
- 體外診斷中交聯劑的用途與選擇
- 優異的化妝品級水溶性β-1,3-葡聚糖
資訊文章
您的瀏覽歷史